Introduction Procurement of enough CD34 + cells (CD34 +) by apheresis is essential for the successful engraftment of peripheral blood stem cell transplantation (PBSCT). To determine the processed blood volume of apheresis to obtain a certain number of CD34 +, collecting efficiency 2 (CE2) is generally used, which is calculated from the total amount of CD34 + in the product (PBSC-CD34 +) divided by pre-apheresis CD34 + in the peripheral blood (PB-CD34 +) and by processed blood volume, and CE2 is determined beforehand from the accumulated data in each apheresis site. However, we sometimes experience cases in which collected amount of CD34 + are far from our expected values. It is probably because of the introduction of various CD34 + mobilization methods, new automated blood cell separators and protocols. Hence, we investigated thoroughly the kinetics of CD34 + in PBSC collection.
Patients and Methods PBSC collections in 55 subjects, including 46 patients undergoing autologous PBSCT and 9 healthy allogeneic PBSCT donors, were analyzed prospectively. All patients were administered G-CSF 10 μg/day sc for 4 days and plerixafor 0.24 mg/kg sc at 22:30 on day 4, and PBSC collection was started from 9 am on day 5. Donors were given G-CSF 10 μg/day for 4 to 5 days until 2x10 6/kg (pt's body weight) CD34 + were collected. Apheresis was performed using Spectra Optia ® in CMNC mode. The apheresis time was adjusted depending on the CD34 + yield. CD34 + was analyzed by flow cytometry according to the ISHAGE protocol. Peripheral blood samples were obtained before, and every 1-1.5 hour after the start until the end of apheresis. Samples were also taken from the apheresis collection bag at the same timings as PB.
Collecting efficiencies were calculated as follows:
CE1(%) = [collected CD34 +]x100/([prePB-CD34 +]+[postPB-CD34 +])/2 x [processed blood volume]).
CE0(%) = [collected CD34 +]x100/sum of ([prePB-CD34 +]+[postPB-CD34 +])/2 x [processed blood volume]) in each section (*CE0 was defined in this particular study).
This study was conducted as a part of the prospective study, which was approved by the Ethics Committee of Keio University School of Medicine.
Results More than 2x10 6/kg CD34 + were collected in the first day in 48 subjects (87%). The median processed blood volume and time was 5575 (range 3873-10903) mL and 185 (range 120-292) min, respectively.
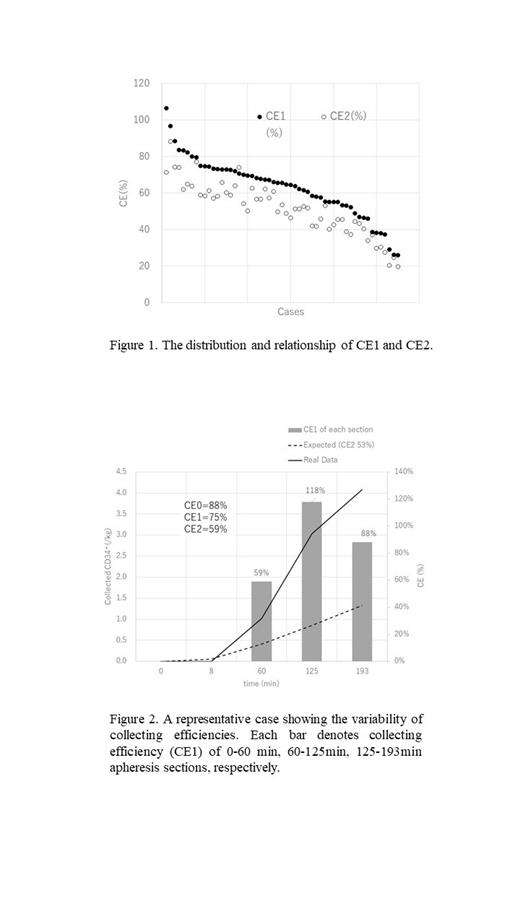
CE2 was calculated as a median 53% (range 20-88) from this cohort, but it varied markedly; CE2>70% in 6 cases, and <30% in 4. On the other hand, CE1 was median 65.5% (range 26.2-106.4%), and it also varied markedly; CE1>80% in 6 cases, and <40% in 7. CE1 and CE2 correlated strongly with each other (r=0.93), but CE1 was higher than CE2 in all cases but one; median 124(range 95-149) % (Fig.1). The main reason why CE1 was always higher than CE2 was probably because PB-CD34 + concentration was always declining during apheresis, which might be more influenced in plerixafor mobilization where CD34 + concentration changed in an hour-depending manner. In some apheresis cases with extremely low CE, it was revealed that cell aggregates containing myriads of platelets might have blocked the outlet of the collection kit, which was difficult to be recognized during apheresis.
CE1 in each apheresis section also changed markedly during each apheresis and CE2 was not helpful for predicting optimal apheresis time (Fig. 2). In the first 10-20 minutes until the interphase formation, a real collection was not yet started so that CE1 in the first 1 hour became low. In the following sections, collection was supposed to become relatively steady, where CE would be influenced largely by interphase stability. In the last part of collection, CE tended to be relatively high for unknown reasons. CEs varied markedly during the whole collection periods.
Conclusions Because multiple factors are associated with CE variability, CD34 + yields were sometimes far from the expected values calculated from prePB-CD34 + and CE2. Therefore, enumeration of CD34 + in the product during apheresis is recommended for secure and effective collections.
Disclosures
Yamazaki:Sysmex Corporation: Research Funding. Kataoka:Nippon Shinyaku: Honoraria, Other: Scholarship; Pfizer: Honoraria; Bristol Myers Squibb: Honoraria; SymBio Pharmaceuticals: Honoraria; Takeda Pharmaceutical: Honoraria, Other: Scholarship, Research Funding; Janssen Pharmaceutical: Honoraria; Kyowa Kirin: Honoraria, Other: Scholarship; Sumitomo Pharma: Honoraria, Other: Scholarship; AstraZeneca: Honoraria; Chugai Pharmaceutical: Honoraria, Other: Scholarship, Research Funding; Novartis: Honoraria; Eisai: Honoraria, Other: Scholarship; Ono Pharmaceutical: Honoraria; Daiichi Sankyo: Honoraria, Other: Scholarship; Alexion Pharmaceuticals: Honoraria; AbbVie: Honoraria; Meiji Seika Pharma: Honoraria, Research Funding; Sanofi: Honoraria; Sysmex: Honoraria; Mundipharma: Honoraria; Incyte Corporation: Honoraria; Kyorin Pharmaceutical: Honoraria; Asahi Kasei Pharma: Other: Scholarship; Otsuka Pharmaceutical: Other: Scholarship, Research Funding; Shionogi: Other: Scholarship; Teijin Pharma: Other: Scholarship; Japan Blood Products Organization: Other: Scholarship; Mochida Pharmaceutical: Other: Scholarship; JCR Pharmaceuticals: Other: Scholarship; Nippon Kayaku: Other: Scholarship; Chordia Therapeutics: Research Funding. Tanosaki:Kyowa Kirin: Honoraria; Ikeda Rika: Honoraria; Janssen Pharmaceuticals: Honoraria; Bristol Myers Squibb: Honoraria; Becton Dickinson: Honoraria; Sysmex: Honoraria; Boston Consulting Group: Honoraria; Novartis Pharmaceuticals: Honoraria; DAI-DAN: Research Funding.